Assistance with Taiwan FDA Regulations
Our Service of Registration/Certification and Specific Industry Surveys of Medical Devices, Cosmetics, and Foods/Functional Foods (Is premarket approval from the Taiwan FDA required before your products can be marketed in Taiwan?) in Taiwan.
Food Drug Administration in Taiwan of ROC (TFDA) is the competent authority of general foods, functional foods, cosmetics, and medical device surveillance, approval and registration/certification in Taiwan. Staff of SCG , who had been trained and certificated by Taiwan FDA, has the expertise and the experience to help companies to list their medical device products and to comply with the regulations. We are also familiar with Functional Foods, Tablets & Capsules, Cosmetics and Advertising Regulations as couple of our staff are qualified official professionals, certificated by The Examination Yuan of ROC as Legal Qualification of Food Professionals.
Health (Functional) Food Registration/Certification in Taiwan
When Health Food Regulation was published in 1999 in Taiwan by TFDA, health food is a legislative noun since then. Company must get certification of the product first before they put the product with the functional claim on the market. The regulation defines the advertising statement of the product as well. If the applicant company is not familiar with regulations, she could hire the professional assistance.
Food Regulatory Consulting Services
- Ingredients and Label Review
- Compliance review/Compliance strategy
- Health Food Registration/Certification
- New Additives
- New Ingredients
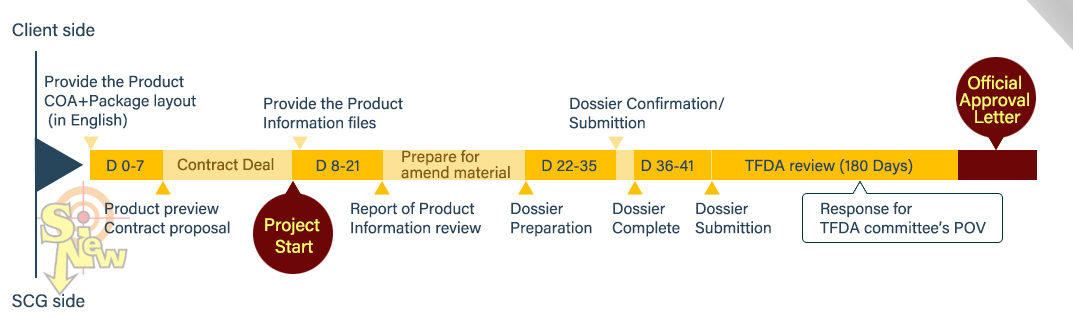
[Free request on demand Health food registration flow chart Track-1]
[Free request on demand Health food registration flow chart Track-2 only for fish oil and anka]
www.e-sinew.com
contact us via whatsapp
