The unique non-conventional foods regulations in the Greater China – especially for Taiwan and Mainland China
In terms of so called “unique non-conventional foods ” including dietary supplements, functional foods, pharmaceuticals, capsule and tablet foods, health foods, infant formula foods, elderly foods, foods for special medical purposes, foods for sport nutrition etc., will be regulated in special regulations both in Taiwan by Taiwan FDA/TFDAand Mainland China by SAMR/CFDA. CFDA employs the term as special foods.
- Capsule and tablet foods : means all foods that in type of capsule or tablet which consumers are not able to expect what ingredient(s) will be.
- Foods for special medical purposes : means those foods design for diabetic, cancers, chemotherapy, and so on.
Some international companies will choose Taiwan as a trial for the huge market in Mainland China. To get the food regulation compliance in Taiwan (Taiwan food compliance) become a need recently. As mentioned above, all the unique non-conventional foods will need to get approval letter or registration number before you can export to Taiwan. The most confusing one is the category of capsule and tablet foods. You need to register with Taiwan FDA even you only have common food ingredients in it. You are even not able to sell it as general food in Mainland China. This means that officers in both areas do not believe general food/common food/regular food/conventional food should be in a capsule or tablet form.
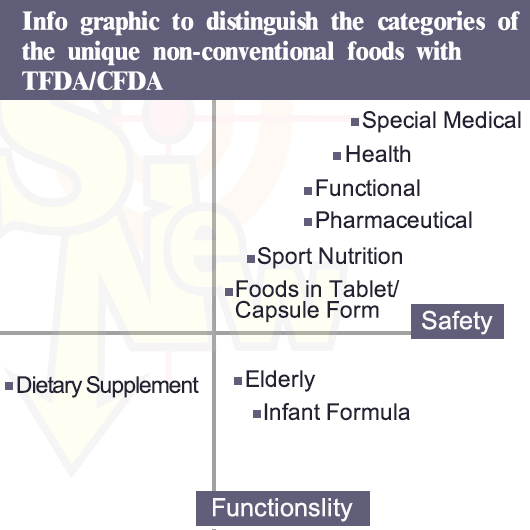
You will need enough scientific supporting documents for these unique non-conventional foods . The test report done in the original country can be the reference evidence when you deal with Taiwan FDA while they only accept the test done in Mainland China as you deal with SAMR/CFDA.
Taiwan FDA regulation compliance
SAMR/CFDA regulation compliance
